Wrapping up our discussion of the ICD-10-CM and PCS code updates released on Oct. 1, in this article we will examine some PCS-related changes. I encourage you to take the time to review all the new codes and changes in detail.
First, there were no guideline changes for PCS with this update. Looking at procedures that are categorized as operating room (OR) procedures versus non-OR procedures, however, we did see some changes.
We can note that there were no procedures that went from being designated as an OR procedure to a non-OR procedure. We did, however, notice some procedures that did the opposite.
Laparoscopic biopsies of the large intestine will now be included in the OR procedure list. This includes excision of both the right and left large intestine, as well as the transverse, descending, and sigmoid colon.
Another biopsy procedure that had a change from non-OR to OR is laparoscopic gallbladder biopsy. This would be code 0FB44ZX – Excision of Gallbladder, Percutaneous Endoscopic Approach, Diagnostic. This will now be designated as an OR procedure for some MS-DRGs. I encourage you to review the update for the MS-DRGs included.
The last OR designation change is for laparoscopic biopsies of the pancreas. This would be code 0FBG4ZX – Excision of Pancreas, Percutaneous Endoscopic Approach, Diagnostic. This will now be designated an OR procedure for some MS-DRGs, as detailed in the update.
We also had quite a few additions to the new technology codes. This is an area I always find interesting and exciting to investigate, as it pertains to new developments.
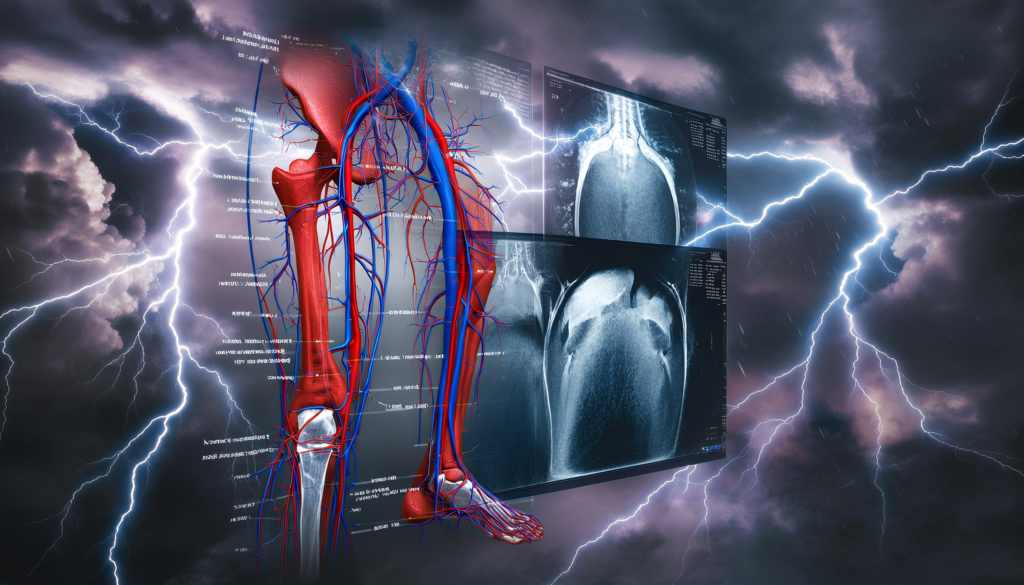
Some of the new codes in New Technology Group 10 include a set of Everolimus-eluting resorbable scaffolds, which are used in the tibial and peroneal arteries. These kinase inhibitor eluting devices help keep those below-the-knee arteries open.
There is also a set of new technology codes for paired titanium cages in spinal fusion. For fusion of the ankle and tarsal joints, new technology for a gyroid-sheet lattice-design internal fixation device is added. Under Monitoring, there is an adhesive ultrasound patch that monitors blood flow. These are just a few examples, so please review the update for all the details on the new technology additions.
Finally, I want to note two new add-on payments for 2025. While the add-on payments in the update ranged from around $100 per payment to tens of thousands of dollars per payment, you would not want to miss out on the next two.
They both are used in the treatment of sickle cell disease. The first is Lyfgenia, a stem-cell-based gene therapy that is administered as a single-dose IV infusion. The add-on payment here is $2,325,000.
The second is Casgevy, also a one-time gene therapy treatment that is individualized for each patient, using edited blood stem cells. There are some CM and PCS code assignment requirements with this one, and the add-on payment is $1,650,000.
Hopefully this overview of the new changes has inspired you to review all the 2025 changes in depth.